In December 2023, the FAMHP launched www.PharmaInfo.be. A website tailored to citizens and patients had long been an idea of the FAMHP. As part of a multi-year patient information plan, this project became much more concrete, and thanks to the explicit support of the Minister for Health, it gained momentum. In the autumn of 2022, the FAMHP was officially able to begin development. It was the Proper Use Division that had to bring the project to fruition in just under a year.
Sarah De Clercq, Head of the Information Entity at the FAMHP, describes the process. “The final result is very similar to what we envisioned at the start, but we were able to refine a great deal during development. We listened to users’ needs and tried to respond to them. We received some valuable feedback from patient organisations, the Belgian Centre for Pharmacotherapeutic Information (BCFI/CBIP), the Belgian Pharmaceutical Association (APB) and from our colleagues at other government agencies. This was important because, although it was the FAMHP’s initiative, PharmaInfo is also a gateway to their ‘specific’ information.”
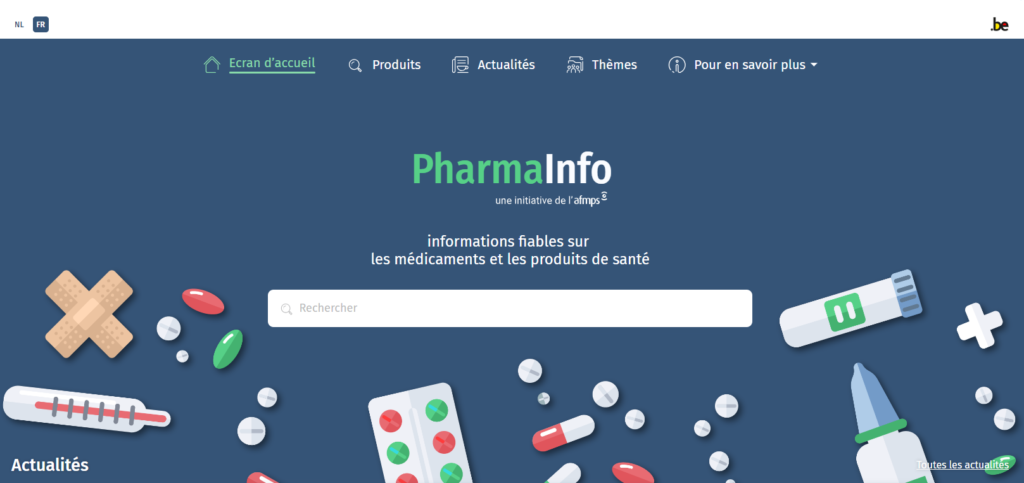
Versatile and patient tailored
The most important thing about PharmaInfo is the search engine. Type in the brand name or active ingredient of a medicine and you will find information on what packaging is on the market in Belgium, whether the medicine is available, what the price and reimbursement are, whether a prescription is required, as well as documents such as the patient information leaflet or additional materials for patients. For some active ingredients, you will also receive a special information sheet containing the most important elements for patients: practical information about the form of administration, how to take the medicine, when, for how long, at what time, etc. Whether you can stop treatment just like that and what to do if you forget a dose. What are the side effects, can you drive a car, what if you are pregnant or breastfeeding, etc. Each sheet is carefully structured so that you can search specifically for the information relevant to you. One hundred sheets were available at launch, and since then, more have been added every month.
FarmaInfo not only provides information about specific medicines, but also useful, general information. For example, what to do with expired medicines, how to report side-effects or how to find a pharmacist or (dental) doctor on duty. Finally, PharmaInfo keeps you up to date with the latest developments through news releases that focus on what patients need to know or do. This keeps patients informed and involved.
Reliable and understandable
In times of fake news and disinformation, it is extremely important to offer citizens the right information so that they can actively participate in their own treatment. Laura De Meester explains. “Reliable and understandable are really the two main principles we always keep in mind. The information you can find on FarmaInfo is based on reliable sources. First, of course, the patient information leaflet, which contains the most up-to-date information about the medicine, but also the BCFI/CBIP’s drug repertory, which guarantees independent scientific information and is based on relevant international sources. But all that correct information is worth nothing if the patient cannot understand it. This is why we use a B1 level. A B1-level text consists of easy words that almost everyone uses. We avoid medical jargon or difficult sentence structures as far as possible. We want everything to be clear to everyone.”
Sarah De Clercq adds to this. “Reliable and understandable also goes beyond what we write ourselves. Fortunately, we don’t always have to start from scratch. For example, the independent Health and Science website developed by the Belgian Centre for Evidence-Based Medicine (CEBAM) has done a very good job of describing diseases and conditions in patients’ language. We make eager use of that and link through to it when we can. Patients also need not fear that we are promoting particular medicines. No, we write our sheets starting from the active ingredients and also provide information on non-medicated treatments, such as in the ADHD care pathway or sleep medicines and sedatives.
But how to select which medicines or topics to write about? Ann Van Den Broucke: “Rest assured, we don’t just decide by ourselves what information we’re going to put out there as a priority. We base it on the patient’s needs. First, of course, we look at what people themselves enter into the PharmaInfo search engine or the FAMHP website and the questions they send us by e-mail. We also receive regular feedback from patient organisations.
Besides that, we review the press or parliamentary inquiries we receive at the FAMHP. Finally, other internal departments can also tell us what’s going on in their fields. And of course, at the Proper Use Division, we also have our own expertise for pushing forward certain topics depending on the time of year, such as vaccinations during the winter season or travel pharmacy just before summer.”
Ann Van Den Broucke
Besides that, we review the press or parliamentary inquiries we receive at the FAMHP. Finally, other internal departments can also tell us what’s going on in their fields. And of course, at the Proper Use Division, we also have our own expertise for pushing forward certain topics depending on the time of year, such as vaccinations during the winter season or travel pharmacy just before summer.”
Not just medicines
The goal of FarmaInfo is to help patients with all types of products designed to support our health. Therefore, there is also information on medical devices. Anaïs Fauche was responsible for that part. “The information on medical devices is more limited because it involves hundreds of thousands of different types of products covered by differing legislation. It’s far more difficult to make the same information – such as an patient information leaflet, manual or the price – available for all resources. However, you can search by the name of a product to find out whether it’s a medical device and who is marketing it. But we mainly focus on information sheets according to the type of product. These include for example thermometers or hearing aids where we cover key things patients need to know, such as precautions, risks or when their use is discouraged.”
Positive start and PharmaInfo continues to evolve
In the first two weeks after the launch, thanks to a successful social media campaign and a great deal of press coverage, PharmaInfo had already had more than twenty thousand visitors. Feedback from individual patients, patient organisations and other FAMHP stakeholders was also positive.
The intention is for the website launched in late 2023 to continue to expand not only in terms of content – some new features will be added Ann Van Den Broucke explains: “From the beginning, we made sure that the website was flexible enough to include information on other types of products too, such as food supplements or cosmetics. We’re now looking at how to best process the information available to the government that can be useful to citizens. But the idea remains the same. A high-performance search engine will guide citizens to all the information about the product in question. In addition, we remain open to what patients want to see and are actively seeking out what we can add ourselves. What can be improved, what can be expanded, where is there a need?”
You will eventually see PharmaInfo popping up in other applications such as ‘Myhealth’ or ‘Mymedicines’. Ideally, for example, when a patient views the summary of open prescriptions in those applications, he or she would have a direct link for each medicine to the relevant information in PharmaInfo. This should also be the case the other way around: from PharmaInfo, you should be able to report an adverse drug reaction with the most important details about the drug already entered.

Laura De Meester – Sarah De Clercq – Anaïs Fauche
Our FAMHP experts
This project is coordinated by the Proper Use Division. Ann Van Den Broucke provides management support, but over the years has become the expert who helps implement innovative applications such as PharmaStatus, the medicines database and the internal Medicinal Product Management system.
Sarah De Clercq is Head of the Information Entity at the Proper Use Division. There, she works with Laura De Meester (for medicines) and Anaïs Fauche (for medical devices) to provide the correct content in understandable language as found on PharmaInfo.




